Sodium-ion batteries face uphill struggle to beat lithium-ion on cost
A new Stanford University study finds that there are several several key routes that sodium-ion battery developers can take to compete on price, specifically against a low-cost variant of the lithium-ion battery known as lithium iron phosphate (LFP).
Sodium-ion battery could charge in several seconds
Researchers at the Korea Advanced Institute of Science and Technology (KAIST) have identified a high-energy, high-power hybrid sodium-ion battery capable of charging in just a few seconds. The system integrates anode materials typically used in batteries with cathodes suitable for supercapacitors.
Weekend Read: A battery worth its salt
While lithium ion battery prices are falling again, interest in sodium ion (Na-ion) energy storage has not waned. With a global ramp-up of cell manufacturing capacity under way, it remains unclear whether this promising technology can tip the scales on supply and demand.
Weekend Read: Hanging in the balance
Battery storage revenues in Britain are well below historic highs but an appetite for storage capacity remains. Electricity system-operator modernization, increased competition, and new opportunities could all shape the future of British battery energy storage systems (BESS’).
New alkali metal-chlorine battery promises 6x energy density
Scientists in the U.S. discovered a promising new battery chemistry based on chlorine and table salt. Batteries based on this chemistry can achieve at least six times the energy density of today’s lithium-ion batteries, according to the group that created it. The prototype battery could already be suitable for small devices such as hearing aids, and with further work could be scaled up to larger applications.
Cathode discovery ‘opens up new pathways’ for calcium-ion storage
Scientists in South Korea tested a new cathode material as part of a calcium-ion battery (CIB), achieving some impressive results. The material retained more than 90% of it initial capacity after 500 cycles, alongside some of the best performance results seen so far for this technology. The scientists say that this discovery opens up “an unexplored pathway toward the realization of stable and high-power cathodes in CIBs.”
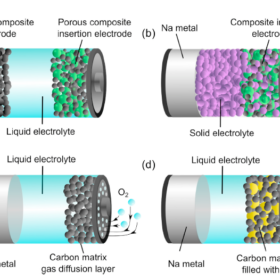
A stable sodium battery, without the anode
Scientists in the U.S. demonstrated a sodium-ion battery with no anode, that retained 99.93% of its initial capacity per cycle. Their design was able to overcome many of the stability issues associated with using ‘pure’ alkali metals in batteries, thanks to carefully minimizing water content in the liquid electrolyte.
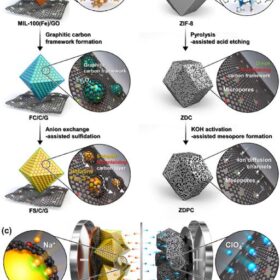
Hard carbon for a high energy sodium battery
Scientists in Japan demonstrated a hard-carbon electrode that can greatly increase the capacity of a sodium-ion battery. With further work on the long-term performance, the discovery could make sodium-ion batteries better able to compete on energy density with their lithium-ion counterparts.
An optimistic – but realistic – perspective for sodium batteries
International researchers have analyzed the potential of sodium-based energy storage and found recent technical advances have arrived faster than those for the lithium-ion batteries which have been studied for three decades. Issues remain, however, before sodium constitutes a complementary option to lithium.
Graphene doping a step forward for sodium batteries
Scientists at Switzerland’s École Polytechnique Fédérale de Lausanne (EPFL) have developed an anode from graphene doped with sodium, which they say could potentially overcome some of the fundamental issues in increasing storage capacity and the lifetime of sodium-ion batteries.