A team of researchers from Imperial College London and Queen Mary University of London have developed a method of harnessing solar energy for hydrogen production.
In the research paper “Enhanced solar water oxidation and unassisted water splitting using graphite-protected bulk heterojunction organic photoactive layers,” available in the journal Nature Energy, the scientists said organic materials such as polymer donors and non-fullerene acceptors have “immense potential” in devices for direct solar hydrogen generation. It goes on to highlight that their use in direct solar water-splitting devices has been so far limited, due to their instability in water and recombination losses at the interface with catalysts.
To address the instability of organic materials in water, the team developed a multi-layer device architecture that integrated a bulk heterojunction organic photoactive layer with a self-adhesive graphite sheet, functionalized with an earth-abundant nickel-iron oxyhydroxide catalyst.
The graphite was found to not only protect the photoactive layer from water-induced degradation but also maintain an electrical connection between the catalyst and photoactive layer without any losses.
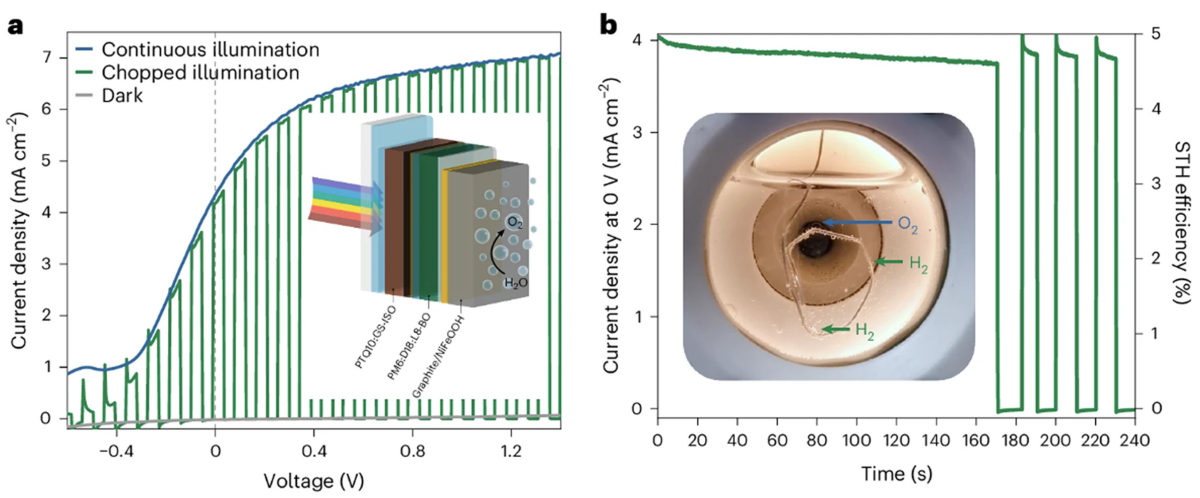
The new device, consisting of fully integrated organic anodes with bulk heterojunction PM6:D18:L8-BO photoactive layers, achieved a photocurrent density of over 25 mA cm⁻² at over 1.23 V versus the reversible hydrogen electrode for water oxidation. The design was also found to demonstrate operational stability for days, unlike earlier designs that degraded within hours.
“Our work demonstrates that high-performance, stable solar water splitting can be achieved using low-cost, scalable organic materials,” said Flurin Eisner, Queen Mary University of London, who led the development of the organic photoactive layers during the project.
“Organic materials are highly tunable in terms of their properties, such as the light they absorb and their electrical properties, which means they can be an extremely versatile platform on which to build various ways to convert sunlight into fuels (such as hydrogen) or even chemicals, emulating natural photosynthesis in plants,” Eisner explained. “This opens exciting new avenues for sustainable fuels and chemicals production.”
The team then prepared monolithic tandem anodes containing organic PM6:D18:L8-BO and PTQ10:GS-ISO photoactive layers, capable of generating hydrogen from water and light without the need for additional electricity. The device reached a solar-to-hydrogen efficency of 5% in a two-electrode photoelectrochemical setup.
The research paper says this result highlights the potential of integrating organic bulk heterojunction photoactive layers for stable, unassisted solar water splitting and “paves the way towards efficiency, stable and unassisted solar hydrogen generation by low-cost organic photoactive materials.”
Salvador Eslava, lead academic of the study at Imperial’s Department of Chemical Engineering, said the result was a record for solar-to-hydrogen efficiencies in organic photoelectrochemical device performance. “The approach leverages the advantages of organic bulk heterojunctions, which offer impressive photocurrents, photovoltages, abundant elements, and ease of processing, and applies them to the electrodes of photoelectrochemical cells,” Eslava added.
The team is now planning to work on exploring improvements in material stability and scaling the technology for industrial use.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.