As understanding of perovskite solar cells improves, scientists are looking ever more closely into the materials to understand the mechanisms at work and identify factors that limit performance, and potential routes for improvement.
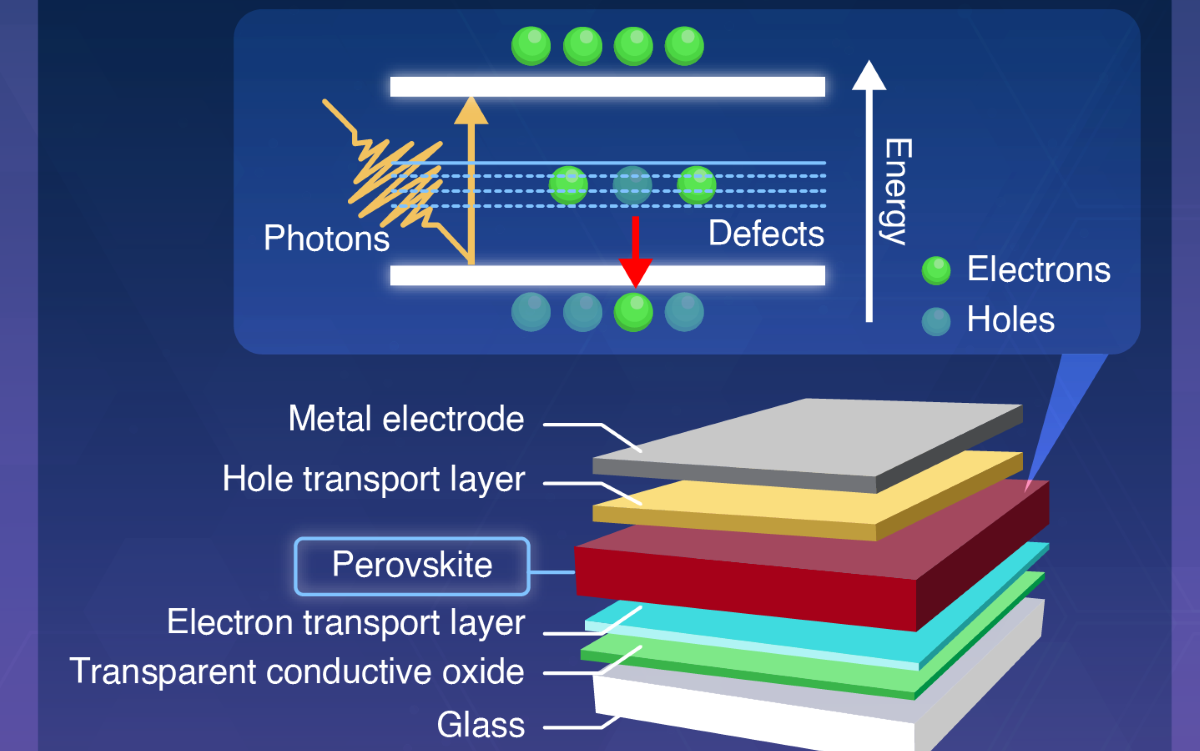
Recent research has identified the interface between the active perovskite material and other cell layers as the source of much performance loss. These interfacial defects promote the diffusion of halide ions across the interface into the charge transporting layer, which adversely affects the long-term operational stability.
With this in mind, an international research team has developed a perovskite solar cell that utilizes crown ethers to power conversion efficiency and moisture-related stability.
The researchers applied density functional theory (DFT) calculations to identify several benzo crown ether candidates with promising ability to bind lead and provide interfacial energetics. The crown ethers were strategically integrated at the perovskite hole transport material interface.
The team found that benzo-18-crown-6-ether (B18C6) was the best ether for interfacial passivation. To establish lead binding performance, the B18C6-treated films were stored in a chamber with a relative humidity of 85% at room temperature without encapsulation. The crystal structure was monitored by X-ray diffraction.
An experimental cell built with this compound achieved a power conversion efficiency of 21.7%, withstanding moisture degradation testing for 300 h at room temperature in 85% humidity with reportedly no lead leakage. This compares an untreated perovskite control device, which reached 20.1% efficiency.
The treatment extended the charge carrier lifetime and lowered the recombination rate between the hole transfer material and the perovskite. The tests “unequivocally affirmed” lead ion capture and suppression of non-radiative recombination”, observed the team.
Analytical techniques used to characterize the samples included time-of-flight secondary ion mass spectrometry (ToF-SIMS), ultraviolet photoelectron spectroscopy (UPS), time-resolved photoluminescence (TRPL), and transient absorption spectroscopy (TAS).
The scientists said that the B18C6 plays a dual role, “efficiently sequestering and immobilizing lead ions through host-guest complexation and simultaneously establishing a robust interfacial passivation layer.”
“The increase in VOC can be contributed to the passivation effect of the B18C6 treatment,” they also noted.
In addition, the fabricated cells exhibited more stable photovoltaic performances after exposure to 85% relative humidity, with efficiency dropping just 20% from the initial measurement while it dropped 80% in control devices.
“So far, the precise cause analysis of how the numerous perovskite solar cell interfacial materials reported contribute electrically and chemically to the improvement of the long-term stability of solar cell devices and modules is lacking, compared to papers on device efficiency,” lead research Ji-Youn Seo told pv magazine.
For that reason, the next steps are devoted to the “development of new interfacial materials based on molecular engineering” and to “systematically analyze the correlation of data to understand what happens at the interface when single devices and large-area modules are exposed to light during operation, including the behavior of electrons-holes and material changes in the deep level.”
The team will continue to use a range of technologies, including X-ray, nano-IR atomic force microscopy for material study and impedance, TAS, TRPL, maximum power point tracking, and intensity-modulated photovoltage spectroscopy (IMVS) for device physics.
Seo indicated the aim to advance towards the commercial production of more stable and larger devices. “We will apply carefully selected interfacial materials to modules to ultimately secure commercialization technology for perovskite modules with commercial-level stability,” he concluded
The details of the study appear in “Interfacial Engineering Through Lead Binding Using Crown Ethers in Perovskite Solar Cells,” published in the Journal of Energy Chemistry. The research team was from Pusan National University, Kyungpook National University, École Polytechnique Fédérale de Lausanne (EPFL), and the University of Fribourg’s Adolphe Merkle Institute.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.