For the energy transition, the electrification of transport, and dozens of other technological areas, the relatively short lifetime of today’s battery technologies is a persistent roadblock.
Cracks forming in the electrodes of batteries are one cause of performance loss over time, and an issue that many manufacturers are looking to engineer out of the batteries using new materials more resistant to cracking. Research published this week, however, suggests that getting rid of cracks in the cathode may have an unwanted side effect.
“Many companies are interested in making ‘million-mile' batteries using particles that do not crack,” explained Yiyang Li, assistant professor of materials science and engineering at the University of Michigan. “Unfortunately, if the cracks are removed, the battery particles won’t be able to charge quickly without the extra surface area from those cracks.”
Conventional wisdom states that the charging speed for batteries should be improved by using cathodes made from smaller particles, since these have a higher surface area to volume ratio, shortening the distance lithium-ions have to travel.
Li and colleagues put this assumption to the test using sophisticated techniques to track the behavior of individual particles as the battery is charged. “Back when I was in graduate school, a colleague studying neuroscience showed me these arrays that they used to study individual neurons. I wondered if we can also use them to study battery particles, which are similar in size to neurons,” said Li.
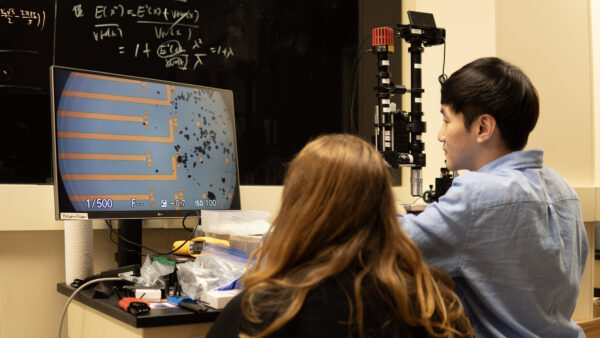
Photo: Jinhong Min, Li+ Research Group, University of Michigan.
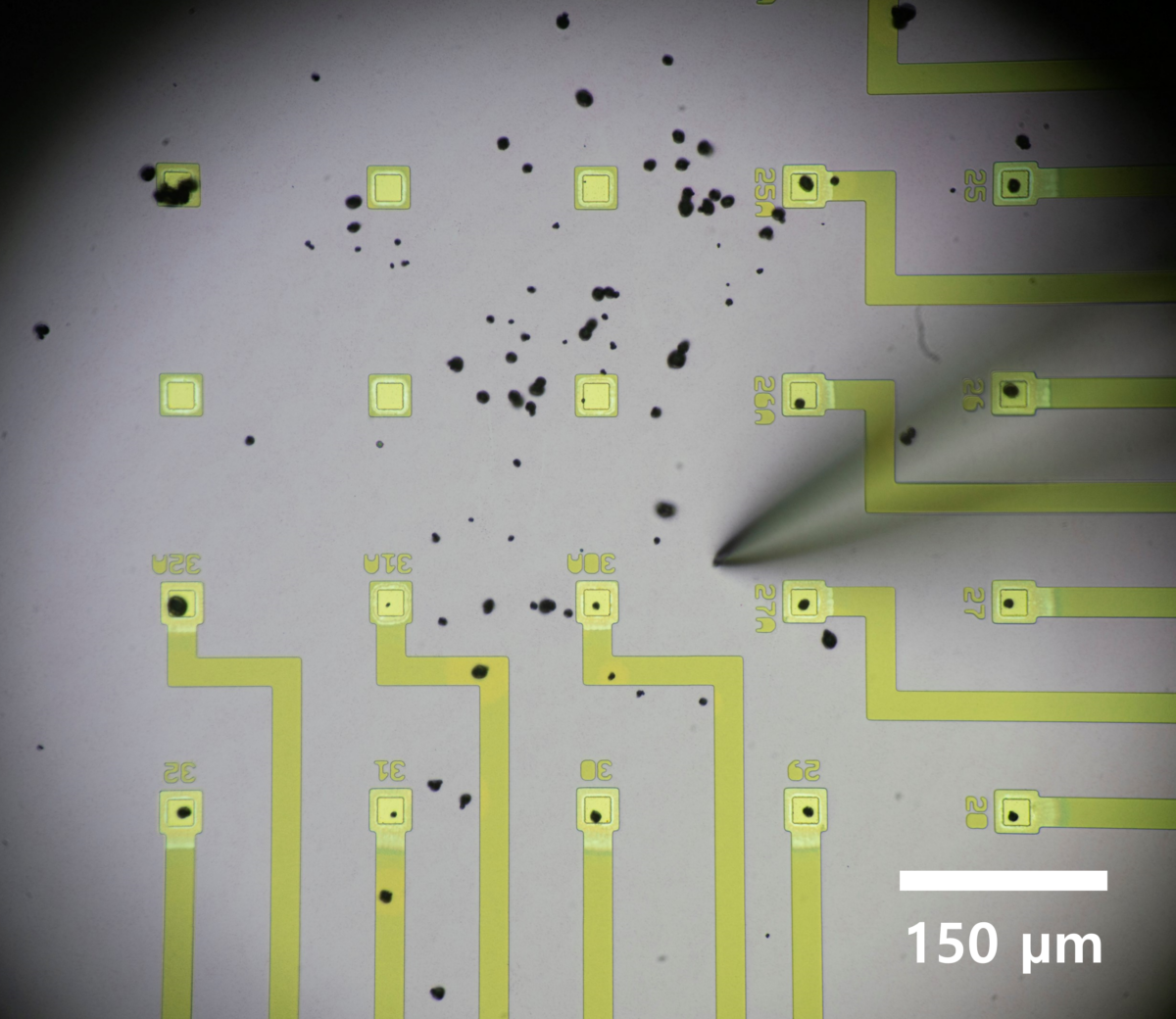
The setup involved a 2x2cm chip containing 100 microelectrodes, onto which the group scattered nickel-manganese-cobalt cathode (NMC) particles. Using a needle which they say is “around 70 times thinner than a human hair,” the scientists could move the individual particles onto an electrode, allowing them to charge and discharge the particles individually. The group worked with NMC “532” material, containing 50% nickel, 30% manganese and 20% cobalt, and say they would expect similar results for any type of NMC cathode.
The experiment is described in full in the paper “Direct measurements of size-independent lithium diffusion and reaction times in individual polycrystalline battery particles,” published in Energy & Environmental Science. The results showed that the charging speed was not affected by particle size.
Li and Jinhong Min, who carried out the experiments, theorize that this could be down to the larger particles behaving more like a collection of small particles when the cathode cracks, Or that lithium-ions are able to move more quickly at the grain boundary. “Our results overturn the dominant picture of lithium transport in the most widely-used cathode material,” the two stated. “If this electrolyte cracking model is accurate, then our results show that intergranular cracking, long believed to be strongly detrimental to cycle life, is in fact essential for the ability of polycrystalline particles to (dis)charge at reasonable cycling rates.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.