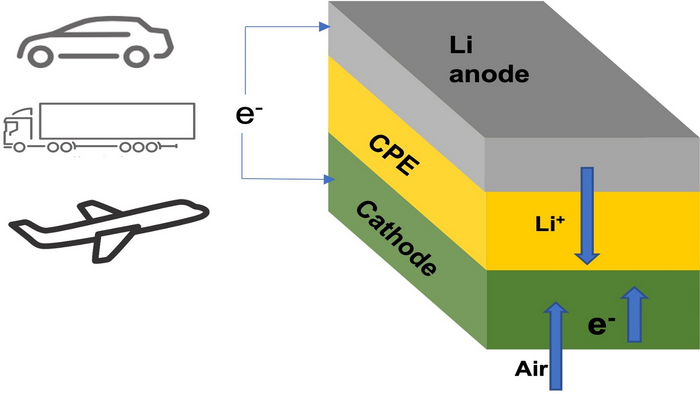
Researchers at the Illinois Institute of Technology and the US Department of Energy’s Argonne National Laboratory have developed a lithium-air battery with a polymer-based solid electrolyte. They claim it offers superior safety and performance.
The solid electrolyte is composed of a ceramic polymer material made from relatively inexpensive elements in nanoparticle form. Specifically, the new composite electrolyte is based on Li10GeP2S12 nanoparticles embedded in a modified polyethylene oxide polymer matrix, which enables chemical reactions that produce lithium oxide (Li2O) on discharge.
In past lithium-air designs, the lithium in a lithium metal anode moves through a liquid electrolyte to combine with oxygen during the discharge, yielding lithium peroxide (Li2O2) or superoxide (LiO2) at the cathode. The lithium peroxide or superoxide is then broken back down into its lithium and oxygen components during the charge. This chemical sequence stores and releases energy on demand.
“The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” said Argonne chemist Rachid Amine.
In the new design, the four-electron reaction is enabled by a mixed ion-electron-conducting discharge product and its interface with the air.
More electrons stored means higher energy density. Theoretically, a lithium-air battery based on Li2O formation can deliver an energy density that is comparable to that of gasoline. It has the highest projected energy density of any battery technology being considered for the next generation of batteries beyond lithium-ion.
“With further development, we expect our new design for the lithium-air battery to also reach a record energy density of 1,200 watt-hours per kilogram,” said Larry Curtiss, an Argonne Distinguished Fellow. “That is nearly four times better than lithium-ion batteries.”
The team’s new design is also the first lithium-air battery to achieve a four-electron reaction at room temperature. In addition, it operates with oxygen supplied by air from the surrounding environment, thus eliminating the need for oxygen tanks, which was a problem with earlier designs.
Past lithium-air test cells have suffered from very short cycle lives. But the US researchers say that this is not the case with their new battery design, as their test cell demonstrated stability over 1,000 charge and discharge cycles.
They discussed their research findings in “A room temperature rechargeable Li2O-based lithium-air battery enabled by a solid electrolyte” which was published in a recent issue of Science.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.