Researchers from France-based Air Liquide working at the company's Innovation Campus Tokyo analyzed all materials that could be used for solid-state hydrogen (H2) storage – including adsorbents, metal hydrides, and chemicals – and considered all potential applications, according to market needs.
In “Nanomaterials for on-board solid-state hydrogen storage applications” – recently published in the International Journal of Hydrogen Energy – the scientists compared the advantages and challenges of physical-based and materials-based hydrogen storage techniques. They looked at compressed H2, liquid H2 or cold/cryo-compressed H2; metal hydrides, metal organic frameworks (MOFs), and chemical H2.
They concluded that physical-based hydrogen storage systems have already reached commercial maturity. Such methods have been used in onboard vehicular applications, but the scientists also acknowledged the challenge posed by are still unable to overcome the current constraints in hydrogen supply chains.
They noted, for example, that carbon composite fiber tanks are still too expensive. They also acknowledged that liquid hydrogen storage is still too costly, even though it enables a higher volumetric density.
“Liquid hydrogen also suffers from unavoidable evaporation, known as boil-off, due to the low enthalpy of evaporation of hydrogen and the difficulties in efficiently managing heat ingress throughout the supply chain,” they explained. “In addition, during the hydrogen liquefaction process, the reaction of converting orthohydrogen to parahydrogen releases heat of around 527 kJ/kg.”
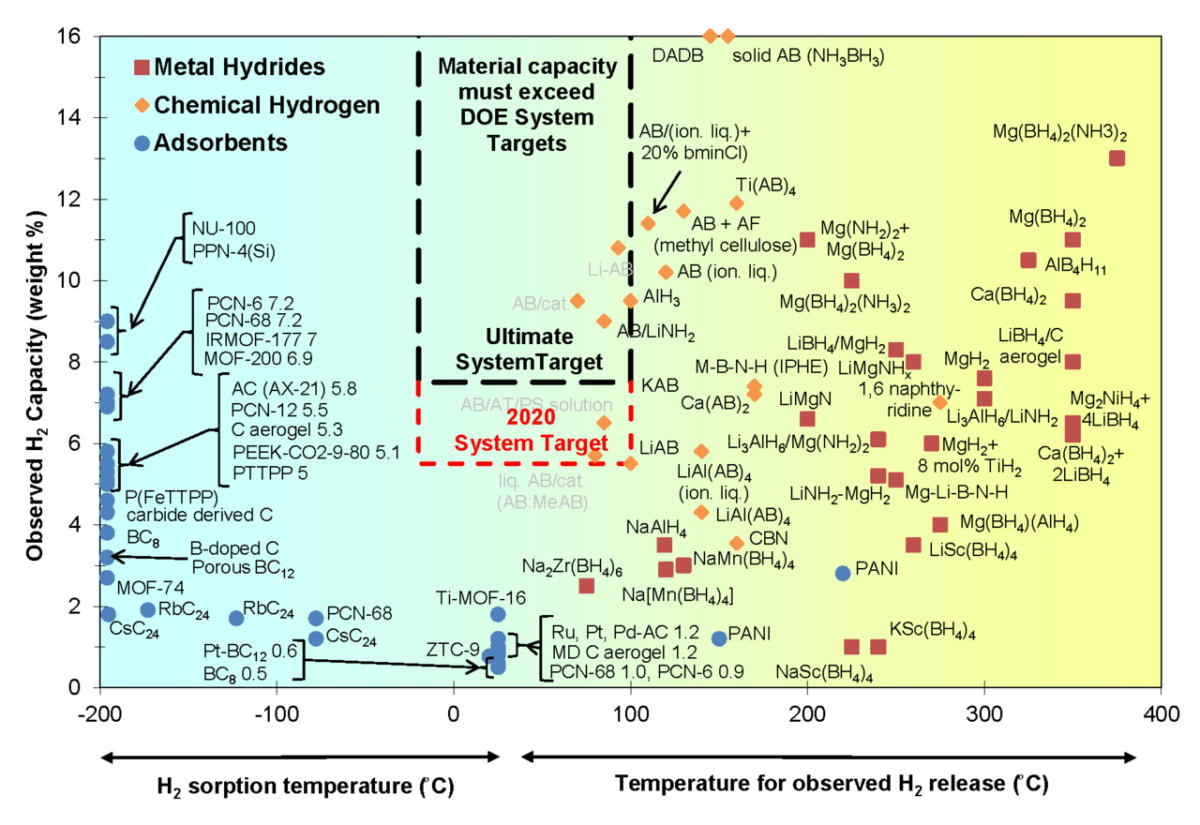
The scientists said that only a few materials-based techniques seem close to a breakthrough.
“Among adsorbent-based hydrogen storage materials, catalyzed graphene-based materials and manganese hydride-based Kubas-type materials seem to be promising. These materials are able to absorb and desorb hydrogen at ambient temperature,” they said, noting that their gravimetric and volumetric density is still not competitive with current physical-based hydrogen storage systems.
The scientists also noted the strong potential of metal hydrides.
“Among metal hydride-based hydrogen storage materials, magnesium and magnesium-based hydrides have been considered as feasible materials to store hydrogen and have attracted huge attention during recent years,” they said. “This is mainly due to the great abundance of magnesium (Mg) in the Earth's crust as well as the high hydrogen gravimetric and volumetric density of magnesium hydride (MgH2).”
The study provides a quick analysis of the technology-readiness level (TRL) of hydrogen storage for onboard applications. The TRL measures the maturity of technology components for a system and is based on a scale from one to nine, with nine representing mature, commercialized technologies.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.