From pv magazine Spain
Spanish inventor José Antonio G. I. has developed a system that is able to generate and store hydrogen on-site from tap water without electrolysis.
“Until now, the most common way to produce hydrogen is by electrolysis. However, this process implies a significant electrical consumption that makes it not interesting from an economic point of view,” he told pv magazine.
The prototype consists of a water tank that is initially filled with water, ferrosilicon, and sodium hydroxide. Hydrogen production begins when a 20 W compressor discharges pressurized air in the lower part of the tank.
“It is the air that causes the reaction between the different chemical components and generates hydrogen,” the scientist explained. “Then, the generated hydrogen leaves through the upper area of the tank to be introduced into an equally pressurized tank. The water in this second tank collects the possible impurities and hydrogen comes out through the upper part of the tank.”
After the hydrogen is purified, it flows via an upper conduit to another tank equipped with closed contacts, a safety valve and an outlet pipe assisted by a solenoid valve.
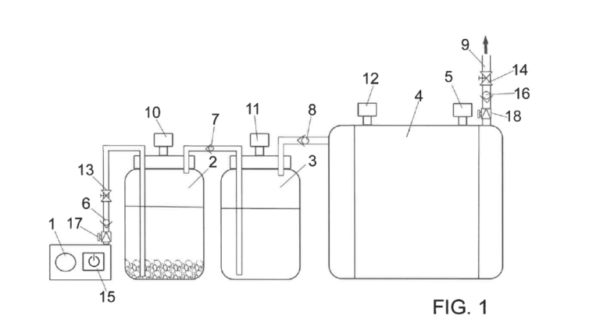
View of the invention, which has been granted a utility model certificate by the Spanish Patent and Trademark Office.
Among the advantages of the device: the hydrogen is produced at the place of consumption, so there is no need for transport. “It can be tailor-made to generate the consumption of a transport truck, or whatever is needed”, the author says. “Currently, we are developing a model with a 220-liter tank that can work with a pressure of 1 kg/cm2 and a flow rate of 30 liters per minute,” González Ibáñez went on to say. “This can generate heating, hot water and electricity for a household or a small business.”
The group is also designing a larger model that can work with a pressure of 750 Kg/cm2 and can reportedly supply a thermal power plant or a container ship.
First compression test, with only two tanks: the generation tank and the rinsing tank, with no safety measures yet.
*The article was updated on July 29 to specify that the two chemical elements added to the first tank are ferrosilicon and sodium hydroxide.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



TANSTAAFL (google it!)
Yeah, it says it costs about 2 pennies per liter of gasoline equivalent of hydrogen.
This looks like a large version of a carbide lantern.
It’s not, the principle is tottaly different.
That doesn’t add up.
Equivalent to a litre of gasoline for .0
15 cent.
Technology has been suppressed from the masses for over 70 years. This could be a small step in the right direction.
Sounds wonderful! I bet a combo setup with solar panels, battery, and this as a baseline backup would be a super way to stay off-grid or fuel a micro-grid.
Do you think the establishment will allow people to have FREE power
Without knowing what other elements are present I can’t put any faith into this. I am not going to take a home video at face value, and neither should anybody else these days. Beyond practical trickery, there’s thousands more ways to deceive us digitally. This coming from a huge fan of the proposed future hydrogen economy.
I’ve seen it work, personnaly. Although you are right, we should never trust what is on the internet, sometimes it works as it should be . A conveyor of news. Maybe if the scientist would described the components he would not be able to continue to perfect his generator without outside “help”.
I agree with Andrew, this has all the sounds of a fake. It needs a lot more investigation by reliable sources. Remember the saying “Extraordinary claims require Extraordinary proof”
They claim 8.33 kWh of energy for for less than 0.02 Euro. 8.33 kWh can be obtained from about 250 grams of hydrogen (33.33 kWh per kg of hydrogen).
After water and fill dirt, concrete is about the cheapest thing you can buy. In volume, can probably get 250 grams of concrete for about $0.02 Euro. I suspect the two undisclosed chemicals cost quite a bit more than concrete.
But let’s say this undisclosed combo of chemicals is as cheap as concrete and the water is free.
Can 250 gram of “something as cheap as concrete” plus any amount of water release 250 grams of hydrogen?
I’m dubious about that. And if the chemicals cost 10x the price of concrete, then it would have to be 25 grams of the chemicals releasing 250 grams of hydrogen. Very much more dubious.
As David Stonier-Gibson said “TANSTAAFL”, and there ain’t any such thing as Cold Fusion…but the process described in this article sure seems like Cold Fusion.
If it sounds too good to be true it probably is…
How can charcoal be of use in the hydrogen process? The reason for my question lies in the fact we are a college with facilities to explore new forms of environmental products, kindest regards Peter
The future is hydrogen and green renewables and is inevitable that the fossile fuels will end. We just have to prepare and start changing before they do end.
Hydrogen will be the fuel the entire world will use as soon as big figures a way to make more money with it than with fossil fuel.
Maybe. But in the meanwhile we can use natural resources to achieve our goals of ending fossil energy before it ends. There are electric cars since de 1884, the first electric car invented was electric and since then 150 years have passed before the momentum gained again. We must use hydrogen, since it’s only surpassed by oxygen and not take 150 years to make it happen.
20Wh in , 8300 Wh out ? That is like perpetum mobile.
No information on how much the chemicals cost, whether they get used up, at what rate they get used up, whether they can be recycled easily and cheaply, and how many kwh of electricity are used.
A lot of information that could have been supplied without naming the chemicals.
Why?
Sounds seriously improbable. Any 12 year old kid could have assembled the equipment in a science class. And photographed it on his phone.
So they’ve put together something that relies on a (probably already well known) chemical reaction to generate H2 from water and “stuff we don’t want to tell you.” I note there is no reference to how much of the reactant is needed, where it comes from, and whether it’s from a renewable source.
This concept is not at all new. It’s potentially useful, sure, but nothing in the description seems revolutionary.
Conservation of energy, or in this case, non-conservation of energy. this is clearly a manifestation of zero point energy
When hydrogen combines with oxygen to form water, a fairly high amount of energy is released. To break apart those chemical bonds and produce hydrogen again requires at least that same amount of energy. If that energy comes from some third substance that is chemically changed, then at least that amount of energy is required to recycle it for use again. Am I missing something?
Correct. Here, the reaction stops when the silicon is used up and fully converted to NaSiO2
I’m constantly amazed by the folks who accept this stuff at face value. Hydrogen and oxygen are held together with atomic bonds — which can only be broken by adding even more energy. This is why the “inventor” stated that electrolysis was of no interest. The same thing applies to chemicals, they will have to supply enough energy to break the atomic bonds. Any chemical with that much energy would be a fantastic fuel and using it to make hydrogen would be a loss because every added step produces another set of losses. You can always find enthusiastic posters backing this sort of thing because schools are so poor and magic thinking is so popular.
Otherwise, it sounds like hogwash. Unless the chemicals are revealed and the process confirmed by others.
Its out there already that Aluminium, Gallium and Water produce Hydrogen. This system shows 3 containers 1 has the water 2 and 3 ?? and pressurised system ? ( the output of A,G,W is Hydrogen, Aluminium oxide mixed with Gallium that is difficult to separate after reaction to produce the Hydrogen. (Chemical processing required to separate the gallium from the Aluminium oxide) the Aluminium is turned to an oxide that would also require a high energy process to return to it Aluminium for re-use.
PS I have done the AGW reaction in my kitchen when water is added to the Aluminium / Gallium mix, Hydrogen and reaction heat is produced along with the AO/G leftover products.
Isn’t this based on a paper published in 1920?
What’s new here?
This method has been around since WWI. Look up Ferrosilicone method of hydrogen production.
Insufficient information. What is the reaction? Are the ferrosilicon and sodium hydroxide both consumed or is one of them just a catalyst? What are these waste products captured in the second tank? What does it cost to dispose of the waste products, and what is the cost in dollars and or energy to replenish the ferrosilicon and the sodium hydroxide. Is the reaction exothermic or endothermic? Without any of that this is just a big tease.
I don’t think they are suggesting you get something for nothing, it’s more about ease of transport. Hydrogen gas is hard to move. Solids like ferro silicon and sodium hydroxide (which could be in solution) aren’t.
Solids are made somewhere else using renewable energy and transported to where the hydrogen needed. So for something like a ship it’s relatively easy to have bunkers with solids (or liquids).
The demo flames burn yellow. That’s either carbon or sodium.
Hydrogen burns blue.
Calm down folks, they already used this technique to fill balloons during WWI. And it was already expensive back then, but also fast. Good idea to further develop, but definitely not “new tech”.
both are consumed resulting in toxic, poision, waste