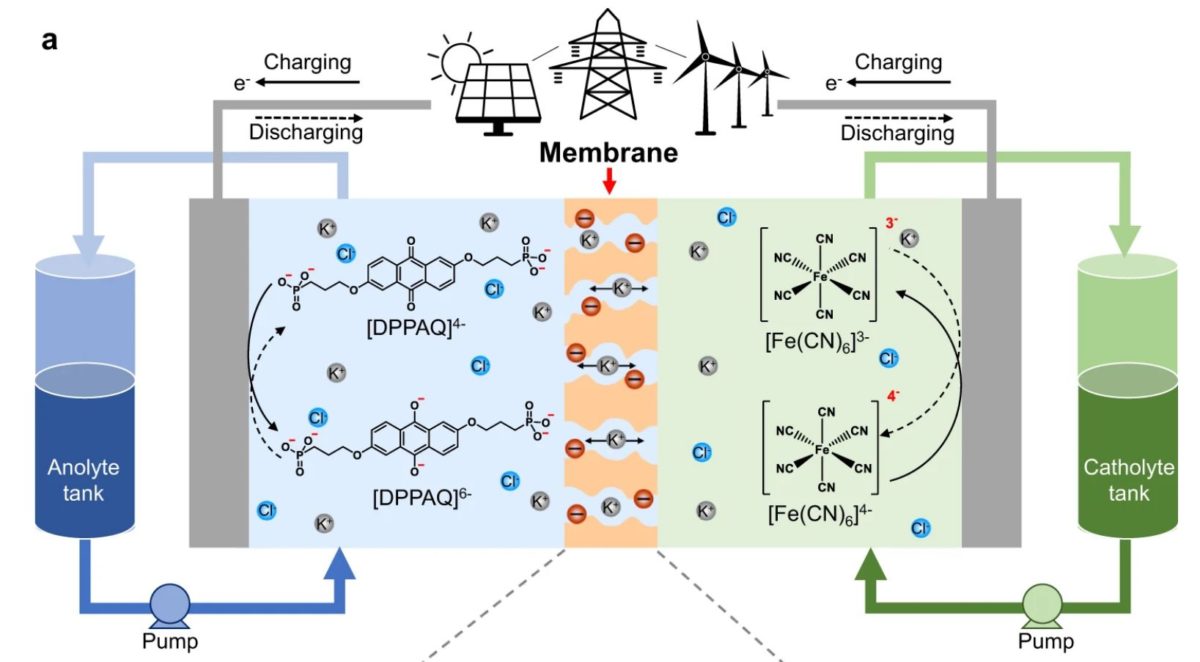
A group of researchers in the United Kingdom has developed a redox flow battery (RFB) based on aqueous organic-based electrolytes which they claim is capable of operating with remarkable stability.
The scientists used ion-exchange membranes with subnanometer ion transport pathways derived from polymers of intrinsic microporosity (PIMs), with either a rigid or contorted backbone, for the novel battery design. They utilized, in particular, a spirobifluorene-based PIM polymer (PIM-SBF), which they claim is able to retain high structural rigidity, block redox-active small molecules and facilitate salt ion transport.
These three actions combined are reportedly able to minimize so-called “crossover.” This phenomenon, which can cause capacity losses that can reach up to 50%, occurs during charging and recharging, when battery electrolyte components cross the membrane in the battery cell and the redoxmers – redox-active molecules that can store energy in the batteries' electrolytes – migrate to the wrong side of the device. “Sulfonated PIM membranes enable rapid transport of small salt cations and inhibits crossover of the large anions and anionic redox-active molecules, resulting in significantly improved performance in comparison to commercial ion exchange membranes and ion sieving membranes from state-of-the-art porous materials,” the scientists further explained, noting that they measure the permeability of salt ions and the crossover of redox-active electrolyte molecules through concentration-driven dialysis diffusion tests.
Through the measurements, the British group found that the battery flow cells based on sPIM-SBF membranes have improved energy efficiency and peak power density compared to reference RFB cells using conventional Nafion 115 membranes. The sPIM-SBF cells were able to maintain high energy efficiency and a very low-capacity decay rate of 0.0335% per day, or 0.0000795% per cycle, for about 120 h or 2,100 charge-discharge cycles. “This is two orders of magnitude lower than that of an otherwise identical RFB using Nafion 115 membrane,” the researchers stated. “It should be noted that these laboratory tests using low concentration of electrolyte resulted in multiple cycles each day, yet the results suggested that PIM membrane showed high rate of capacity retention due to minimal crossover over the testing period.”
The researchers also assumed that if the RFB stacks were operated under ideal conditions, such as constant operating temperature and no oxygen penetration, the capacity loss may come exclusively from membrane crossover. “These data allow the prediction that the highly ion-selective sPIM-SBF membrane would maintain 80% capacity performance of a practical RFB utilizing a high concentration of redox-active materials over 29 years, whereas, the same RFB using a Nafion 115 membrane would maintain 80% capacity performance for only 1.5 years,” they emphasized.
They presented the battery design in the paper “Development of efficient aqueous organic redox flow batteries using ion-sieving sulfonated polymer membranes,” published in nature energy. The research group includes academics from the Imperial College London and the University of Edinburgh. “Our study proves that engineering the subnanometer pores of membranes and enhancing the membrane selectivity substantively improves the performance of this class of RFBs,” they concluded.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.