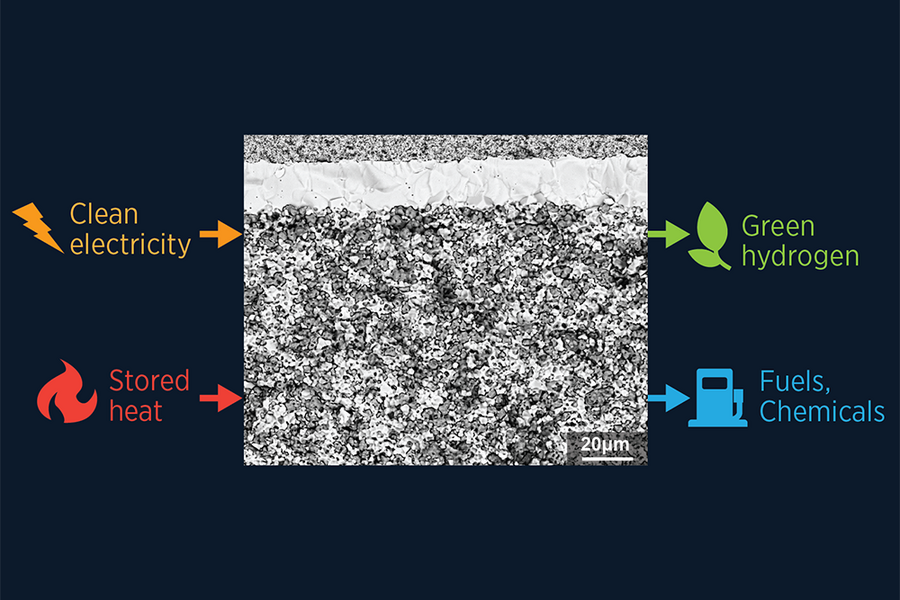
The application of acid for 10 minutes on the top of an electrolyte made of barium, cerium, and zirconium can etch grooves into the surface and remove segregating elements, improving the performance of intermediate-temperature electrolyzers based on protonic ceramic electrochemical cells. Scientists from the Massachusetts Institute of Technology (MIT) have applied the process to address the low proton conductivity of cells. “Their acid-treated cells produced about 200% more hydrogen per area at 1.5 V at 600 C than did any previous cell of its type, and worked well down to 350 C with very little performance decay over extended operation,” the institute's Department of Nuclear Science and Engineering said. The team also found out that the top surface of the dense electrolyte is usually flat. In contrast, the bottom surface of the porous electrode sitting on it is bumpy, and the two come into contact in only a few places. “That precarious interface leads to both structural de-lamination and poor proton passage from the electrode to the electrolyte,” said Ju Li, a paper co-author, likening the method to sandblasting a surface before applying paint to increase adhesion. A growing body of research is currently analyzing the options of electrolyzers working at temperatures between 350 C to 600 C. The interest in electrolyzers working at these “intermediate” temperatures is growing. “Reduced operating temperature enables cheaper materials for the large-scale assembly, including the stack,” said Idaho National Laboratory researcher and paper co-author Dong Ding.
An international team involving Oslo-based CoorsTek Membrane Sciences and Norwegian research entity Sintef has shown that it is possible to preserve the efficiency of proton ceramic reactors also when scaling up from cell to stack level, which usually poses challenges to the distribution of heat and gas flows and electric current. “We demonstrate a 36-cell, well-balanced reactor stack enabled by a new interconnect that achieves complete conversion of methane with more than 99% recovery to pressurized hydrogen, leaving a concentrated stream of carbon dioxide. Comparable cell performance was also achieved with ammonia, and the operation was confirmed at pressures exceeding 140 bars,” the team said. They noted that the stacking of proton ceramic reactors into practical thermo-electrochemical devices demonstrates their potential in hydrogen production. Experts from Valencia's Institute of Chemical Technology (ITQ) participated in the project.
ArcelorMittal has successfully tested the use of green hydrogen in the production of direct reduced iron at its steel plant in Contrecoeur, in the Canadian province of Quebec. “The objective of the test was to assess the ability to replace the use of natural gas with green hydrogen in the iron ore reduction process. During this first test, 6.8% of natural gas was replaced with green hydrogen during a 24-hour period, which contributed to a measurable reduction in CO2 emissions. The green hydrogen used in the test was produced by a third-party-owned electrolyzer and was then transported to Contrecoeur,” the company said. It is assessing the possibility of carrying out further tests in the coming months by increasing the use of green hydrogen. The potential use of electrolyzers to produce green hydrogen in Contrecoeur will mostly depend on the availability of sufficient electricity to power the units.
German Minister of Economic and Climate Affairs Robert Habeck and his Indian counterpart, R.K. Singh, have signed a memorandum of understanding on green hydrogen cooperation. The cooperation activities will include production, processing, and the application and transport of green hydrogen. “Due to the good conditions for renewable power generation, India can become a globally important production location for green hydrogen in the long term,” said the Ministry of Economic and Climate Affairs, adding that the country is interested in selling electrolyzers to the second-largest country in the world. The cooperation agreement aims to establish a task force within the Indo-German Energy Partnership – the so-called Indo-German Energy Forum. The task force would facilitate the exchange of knowledge and experience in regulation, standards and safety procedures. In March, Habeck visited Qatar and the United Arab Emirates. The German government also agreed on a hydrogen collaboration with Norway.
The Canadian province of British Columbia has announced the opening of Vancouver Island’s first public hydrogen-fueling station. The government wants to expand the hydrogen-fueling network to encourage more British Columbians to switch to hydrogen-fueled cars. “Vancouver-based Hydrogen Technology and Energy Corporation (HTEC) has partnered with 7-Eleven Canada to open Vancouver Island’s first public hydrogen-fueling station at an Esso in Saanich. The Saanich station received CAD 500,000 ($389,350) from the Province’s CleanBC Go Electric program and CAD 1 million from Natural Resource Canada’s Electric Vehicle and Alternative Fuel Infrastructure Deployment Initiative,” said the provincial government.
C-Job Naval Architects and LH2 Europe have designed a brand new class of liquid hydrogen tankers. They measure 141 meters and each have a storage capacity of 37,500 cubic meters. “Liquid hydrogen provides unique challenges in ship design and engineering. As a comparison, LNG tankers use ballast water to compensate the loss of weight following delivery to ensure enough draft. As liquid hydrogen is high in volume but 20 times lighter than LNG, this required a unique solution. We have created a trapezium-shaped hull design which creates enough deck space to fit the tanks without the need for ballast,” said Job Volwater, CCO of C-Job. The tankers should be available commercially by the end of the decade. “LH2 Europe aims to have a full liquid hydrogen supply chain ready by 2027,” said Peter Wells, CEO of LH2 Europe. “We plan to initially deliver 100 tons per day (t/d) of green hydrogen and ramp up production to 300 t/d within three years, depending on demand.” The vessel is powered by hydrogen fuel cells.
California-based RIX Industries recently presented the advantages of methanol-to-hydrogen generation technology, underscoring how the shipping industry is increasingly looking at hydrogen. “This new method of producing hydrogen offers greater flexibility to existing ships by repurposing tanks already in use,” the company said. For such designs, hydrogen is not stored, but instead created as needed through a reforming process, allowing the methanol-to-hydrogen propulsion strategy to be distributed across a vessel’s infrastructure. “While hydrogen is an appealing answer industry-wide, its deployment is deeply complicated by cryogenic temperature requirements and/or high-volume, high-pressure storage. However, the ability to generate hydrogen on-demand is breaking through these barriers, tapping into safe, convenient methanol to eliminate complexity and achieve decarbonization,” said Bryan Reid, chief sales officer for RIX Industries.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.

What percentage of a hydrocarbon (Hc) becomes CO² after stripping out the hydrogen? Obviously it’s dependent on the particular hydrocarbon but how does it compare to burning the hydrocarbon?
I just can’t see how reforming Hc’s can be considered green in any sense of the word. It’s a stop gap and disposable technology at best.