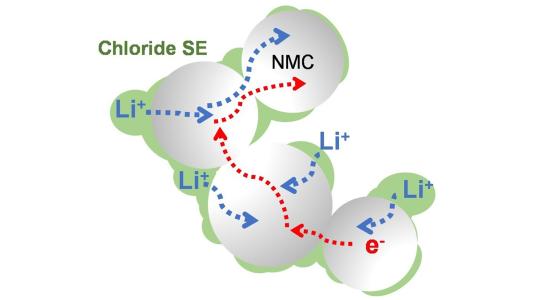
A group of researchers from the University of Waterloo, in Canada, has developed a new solid electrolyte which has demonstrated stable high-voltage operation with capacity retention of more than 4mAh/cm2. This electrolyte, composed of lithium, scandium, indium and chlorine, conducts lithium ions well but electrons poorly, preventing decomposition at high voltages.
With such an electrolyte, an all-solid-state battery can function without significantly losing capacity for more than 100 cycles at high voltage (above 4V) and for 1,000 of cycles at intermediate voltage. According to the researchers, the chloride nature of the electrolyte is the key to the device's stability at operating conditions above 4V.
The device
“Chloride electrolytes have become increasingly attractive because they oxidize only at high voltages and some are chemically compatible with the best cathodes we have,” said Linda Nazar, a distinguished research professor of chemistry at the University of Waterloo and a member of the Joint Center for Energy Storage Research at the U.S. Department of Energy’s (DOE) Argonne National Laboratory. “There’s been a few of them reported recently, but we designed one with distinct advantages,” she added.
Current iterations of solid-state electrolytes focus heavily on sulfides, which oxidize and degrade above 2.5V. They therefore require the incorporation of an insulating coating around the cathode material that operates above 4V, which impairs the ability of electrons and lithium ions to move from the electrolyte into the cathode. “With sulfide electrolytes you have a kind of conundrum — you want to electronically isolate the electrolyte from the cathode so it doesn’t oxidize but you still require electronic conductivity in the cathode material,” Nazar said.
While not the first group to design a chloride electrolyte, Nazar and her colleagues made the novel decision to swap out half the indium for scandium, which proved to be the winning recipe for lower electronic – and higher ionic – conductivity.
One chemical key to the ionic conductivity lay in the material’s crisscrossing 3D structure, called a spinel. The researchers' goal was to load the spinel with as many charge-carrying ions as possible but also to leave sites open for the ions to move through. “You might think of it like trying to a host a dance,” said Nazar, “you want people to come but you don’t want it to be too crowded.”
The professor said it is not yet clear why the device's electronic conductivity is lower than many previously reported chloride electrolytes but it helps establish a clean interface between the cathode material and solid electrolyte, a fact largely responsible for the stable performance even with high amounts of active material in the cathode.
The findings were published in the paper “High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes,” in the journal Nature Energy. The research was funded by the DOE units the Office of Science and the Office of Basic Energy Sciences, with support from Canada’s National Sciences and Engineering Research Council.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.