Among the many different battery chemistries promising a big jump in the capacity, lifetime and cost of energy storage, lithium-sulfur stands out for its potential for strong performance without turning to rare or difficult to obtain materials.
Lithium-sulfur batteries, however, tend to lose their capacity very quickly thanks to an unwanted side reaction that occurs between cathode and electrolyte as the battery cycles. This leads to the formation of polysulfides, which cannot be reversed and quickly causes the battery to shut down. Most of the solutions to this suggested by researchers have focused on working with different electrolytes better able to cycle with sulfur, or changes to the separator film keeping the two components apart.
Scientists led by Drexel University in the US instead decided to focus on modifying the sulfur containing cathode to work better with commercially available carbonate electrolytes. “Having a cathode that works with the carbonate electrolyte that they’re already using is the path of least resistance for commercial manufacturers,” explained Vibha Kalra, a professor at Drexel University who led the research. “So rather than pushing for the industry adoption of a new electrolyte, our goal was to make a cathode that could work in the pre-existing Li-ion electrolyte system.”
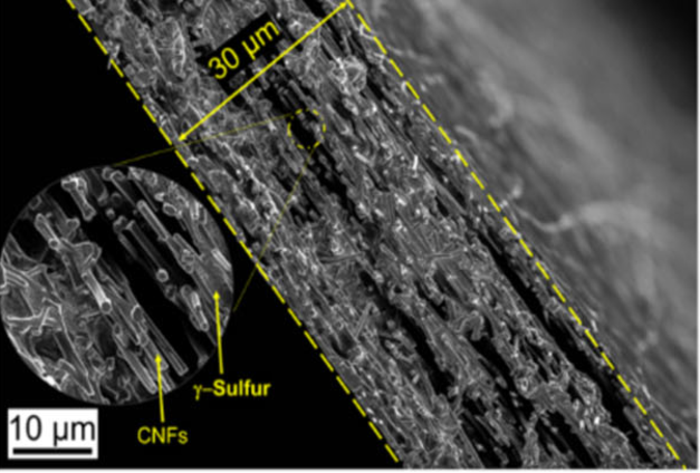
Monoclinic gamma-phase sulfur
The group’s initial approach relied on embedding sulfur within a carbon nanofiber mesh designed to mitigate the polysulfide reaction. While this wasn’t successful, their cathode performed far better than expected in testing, and they set out to explain why. After a lot more testing, the group was able to confirm that during deposition, the sulfur had crystallized in an unexpected way, forming a variation on the element known as monoclinic gamma-phase sulfur.
“At first, it was hard to believe that this is what we were detecting, because in all previous research monoclinic sulfur has been unstable under 95 degrees Celsius,” said Rahul Pai, a doctoral student at Drexel. “In the last century there have only been a handful of studies that produced monoclinic gamma sulfur and it has only been stable for 20-30 minutes at most. But we had created it in a cathode that was undergoing thousands of charge-discharge cycles without diminished performance — and a year later, our examination of it shows that the chemical phase has remained the same.”
The battery is described in full in the paper Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li-S batteries, published in Communications Chemistry. It demonstrated an initial capacity of 800 milliamp hours per gram (mAh/g−1), which had fallen to 650mAh/g−1 after 4000 cycles.
“While we are still working to understand the exact mechanism behind the creation of this stable monoclinic sulfur at room temperature, this remains an exciting discovery and one that could open a number of doors for developing more sustainable and affordable battery technology,”
The group notes that further investigation of the cathode’s surface, the role of carbon nanofibers and potential additives to the electrolyte would be needed for the technology to reach “commercial-grade performance.” And they hope this work will inspire more research into the fundamental behavior of sulfur in various battery types. “This will enable a deeper understanding of the system facilitating the commercialization of Li–S batteries,” they conclude.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



2 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.