From pv magazine Germany
Hydrogen could soon be stored in “nano-chocolates” instead of 700-bar pressure tanks, according to recent research by scientists from Germany's Deutsches Elektronen-Synchrotron (DESY) institute. The researchers claim that they have managed to bind hydrogen with the help of palladium and iridium, before releasing it again.
Palladium, a precious metal, is considered suitable for long-term hydrogen storage. The element absorbs hydrogen like a sponge, according to DESY.
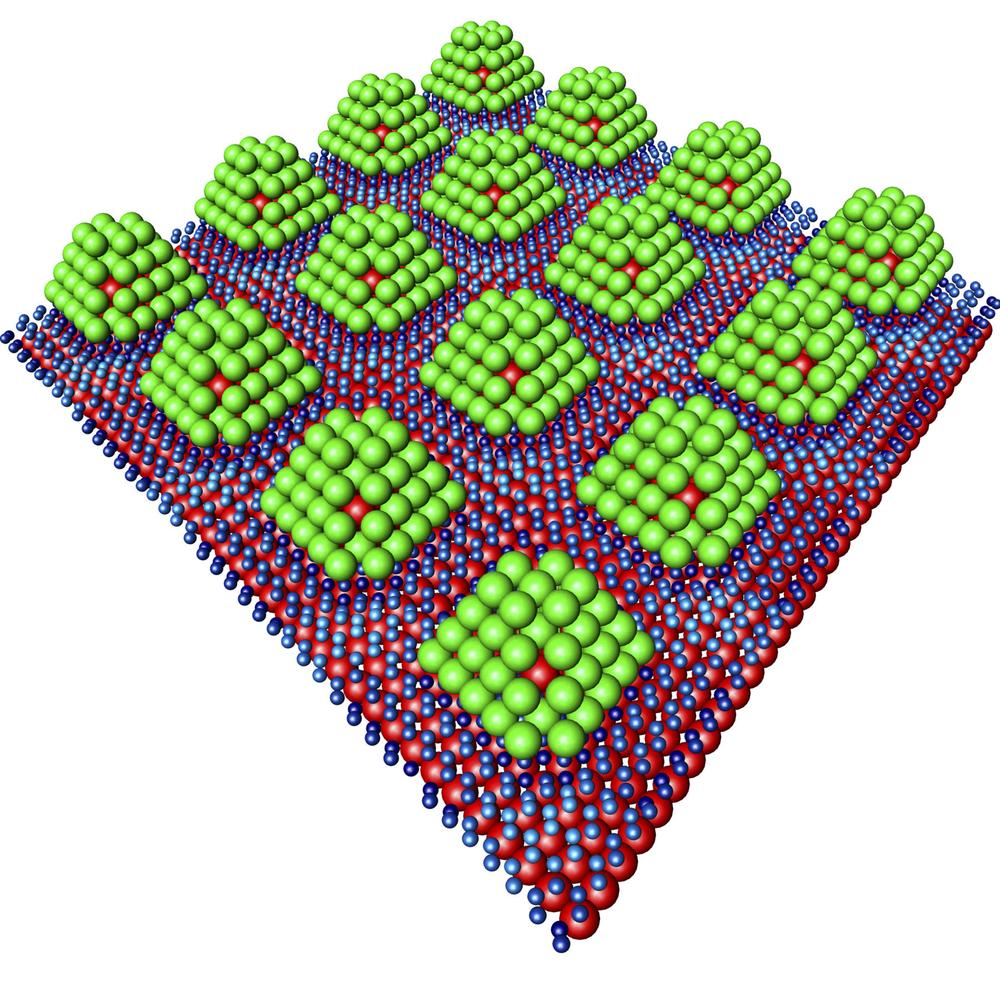
“However, until now getting the hydrogen out of the material again has posed a problem,” explains Andres Stierle, the leader of the research team. “That’s why we are trying palladium particles that are only about 1 nanometer across.”
The special structure of the storage medium has driven comparisons with chocolate pralines. The small particles are built up like pralines and embed an iridium core that provides needed stability. On the outside, a layer of palladium surrounds the core. The palladium-iridium “pralines” themselves are only 1.2 nanometers in size and were fixed onto the graphene at intervals of just 2 nanometers.
Using an X-ray light source, the researchers were able to follow how hydrogen molecules bind to the palladium particles and then loosen again. This enabled them to see that the hydrogen only binds to the surface of the palladium, and that it can be easily removed from the palladium by slightly increasing the temperature.
Conventional hydrogen storage methods require the gas to be liquified or kept in pressurized tanks, at up to 700 bar. This means cooling it down to minus 253 C. However, DESY's proposed approach purportedly requires much less energy.
“Next, we want to find out which storage densities we could achieve with the new method,” says Stierle.
However, the researchers will need to change the carbon medium to do this. In order to enlarge the surface on which the palladium nanoparticles can be arranged and to accommodate the particles in “significant quantities,” the researchers are considering carbon sponges.
They recently published their findings in ACS Nano, a journal produced by the American Chemical Society.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



Hmmm, uses a precious metal. I foresee that we’ll be having thefts of hydrogen storage units from our vehicles to recover the palladium, just like today people steal catalytic converters to recover the platinum.