Getting more out of lithium-ion and other energy storage technologies is the focus for scientists the world over. Batteries are already making a valuable contribution to the energy transition, but there are still plenty of challenges and improvements to be made.
And while a lot of this research is focused on working with entirely new materials that show promise for energy storage applications, squeezing more out of established technologies, and understanding the mechanisms behind their limitations, is a valuable prospect for many. Faster charging presents one challenge for today’s batteries, particularly relevant to electric vehicle applications – and understanding how the higher currents needed for fast charging can cause damage and performance loss within the battery is the focus of recent work from scientists led by Argonne National Laboratory in the United States.
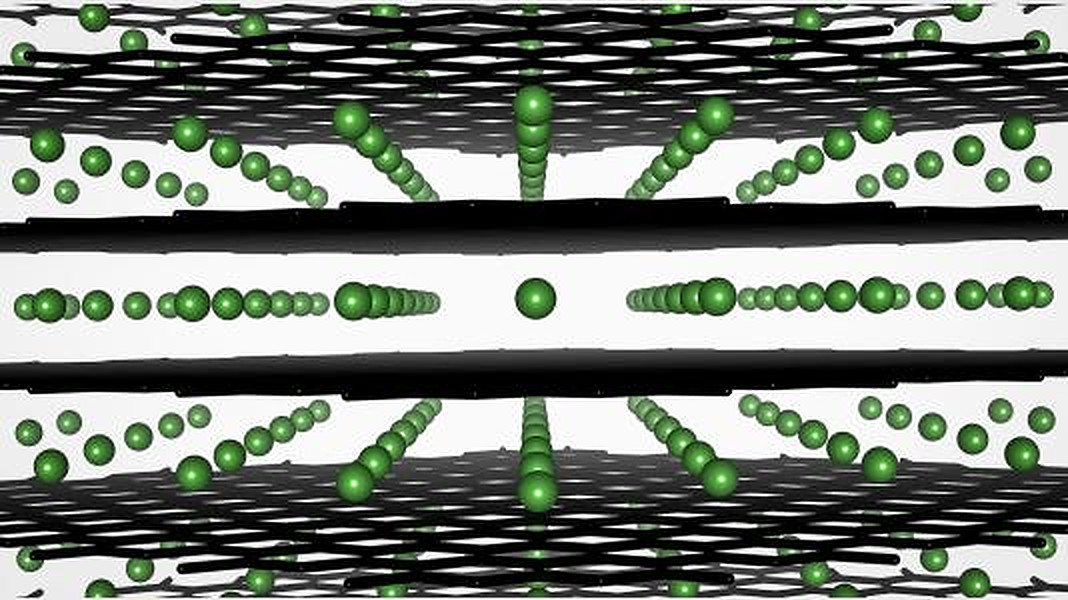
The group took an ‘as prepared’ graphite anode that had not been cycled within a battery or even exposed to an electrolyte, and compared it to another taken from a cell that had been through several cycles of fast charging. Both anodes were examined using sophisticated imaging and characterization techniques, which are described in the paper Increased Disorder at Graphite Particle Edges Revealed by Multi-length Scale Characterization of Anodes from Fast-Charged Lithium-Ion Cells, published in the Journal of the Electrochemical Society.
In addition to plating – where lithium from the electrolyte is permanently deposited on the anode’s surface rather than reversibly stored within graphite particles – the group noted changes to the anode’s structure that further reduced its capacity. “Basically, what we see is that the atomic network in the graphite becomes warped, and this prevents lithium ions from finding their ‘home’ inside the particles — instead, they plate on the particles,” explained Argonne scientist Daniel Abraham. He added that this effect appears to increase the faster the battery is charged, and is visible even after just a few cycles. “The key is to find ways to either prevent this loss of organization or to somehow modify the graphite particles so that the lithium ions can intercalate more efficiently.”
The group suggests that raising the cell cutoff voltage, or increasing the space in the lattice of graphite particles, could be potential solutions, but each would come with its own drawbacks. The improved understanding of the mechanisms behind plating and performance loss, however, should open new doors to researchers looking for solutions.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.