From pv magazine Germany
Munich-based residential vanadium redox flow battery start-up Voltstorage and the Landshut University of Applied Sciences have launched a research project to develop a prototype iron redox flow storage system based on Voltstorage's residential battery.
The proposed iron redox flow (IRF) technology is expected to make the storage system more cost-effective and suitable for applications at very high temperatures.
In the initial phase of the project, which is being funded by Germany's Federal Ministry of Research and Economics, the researchers will develop a residential storage system with a capacity of 8 kWh that is compatible with both solar and wind power installations. At a later stage, they will design a device for commercial applications that should have a storage capacity of 50 kWh.
Voltstorage said the advantages of iron electrolyte consist largely in using pure water, with the device, therefore, non-flammable, even in the event of extreme environmental conditions or malfunctions. Iron, on the other hand, can be 100% recycled, is available in large quantities worldwide, and is also particularly cheap. In addition, the storage systems built with this technology could also be installed at high temperatures, for example in subtropical and tropical regions.
The start-up aims to achieve a levelized cost of storage (LCOS) of around €0.05/kWh with this new battery, which would make iron redox flow technology the cheapest on the market. “Due to the low total costs, the IRF technology creates an ecological alternative, especially for larger, scalable commercial and industrial storage systems,” the company stated.
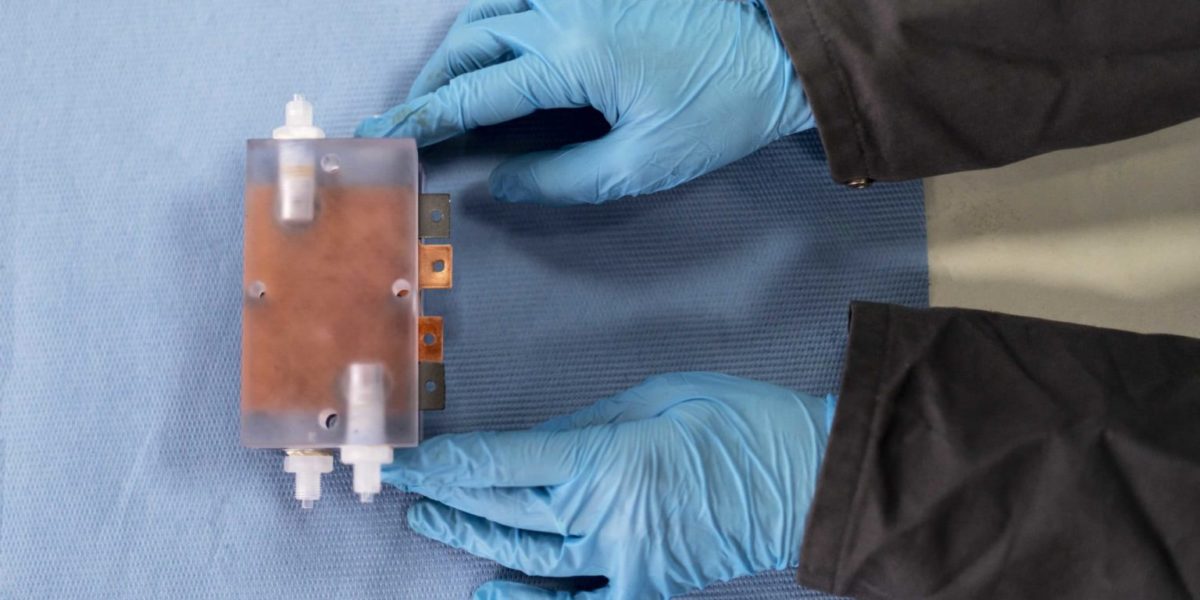
The iron redox flow storage system consists of two battery half-cells separated from one another by a membrane through which an electrolyte fluid is pumped in separate circuits. The electrolyte liquid is said to have been enriched with iron sulfate.
When charging and discharging, ions and electrons are transferred between the two half-cells and the energy is stored in the electrolyte. In the uncharged state, an identical oxidation state prevails in both cells and the reduction in one half-cell, and oxidation of the electrolyte in the other, only takes place during charging. In the negatively charged half-cell, the iron is released from the liquid and adheres to the electrode. The capacity of the battery is proportional to the amount of iron that can be attached to the electrode.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



The Vanadium redox flow battery stores energy in half cells with different charge states of Vanadium. This is not dependent on the surface area of electrode, unlike the Iron flow battery.
Is vanadium not limited, expensive and harmful. They are around for a few years now but have not taken off. But I do like the concept
If residential pv installation size is most popular at about 7.5kw size, an 8 kwh battery is pretty useless. Even a 5kw install with 5 sun/hours is 25 kwh available in just one day. It seems 50kwh storage should be targeted to residential rather than commercial.
Paul, I suspect that 8 kWh battery size is predicated on getting a grid connected home “through the night” a reasonable proportion of the time, rather than storing a high proportion of a day’s generation.
Power throughput and storage capacity are disjointed aspects for redox systems. Capacity is solely based on reactant tank sizing.
Paul Vilella’s comment seems on the mark to me.