Researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory are now trying to understand the mechanisms behind the operation of redox flow batteries (RFBs).
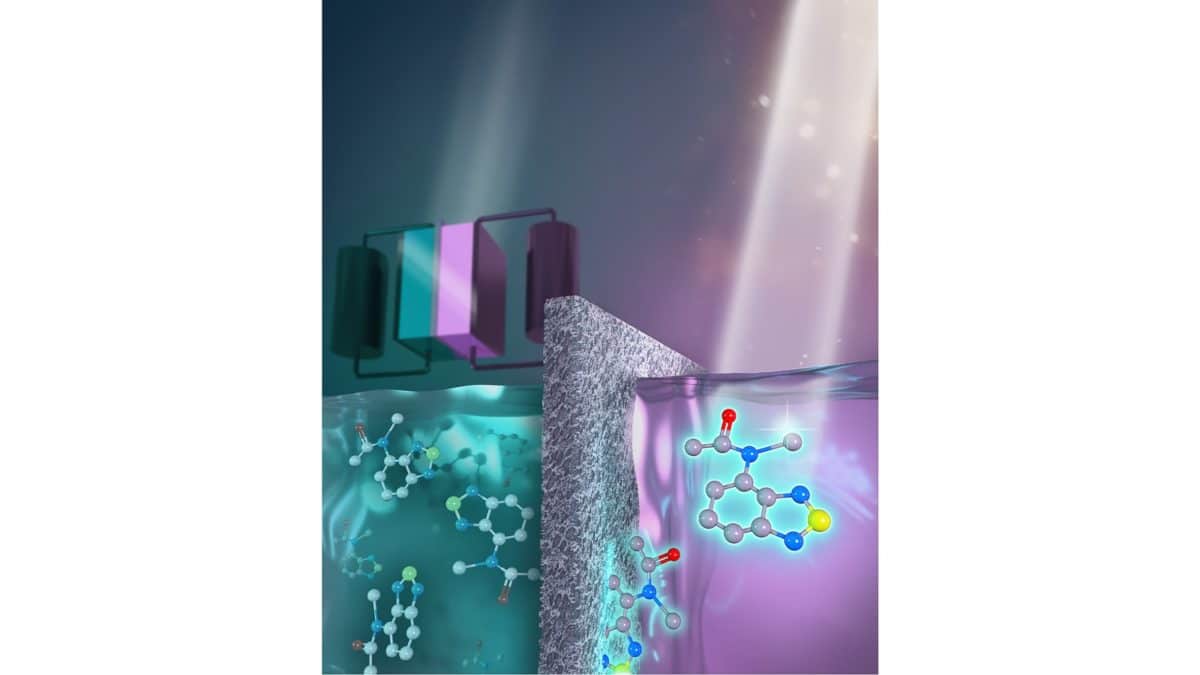
Redox flow storage devices work by storing energy in two separate tanks of fluids. The tanks need different redoxmers, which are redox-active molecules that can store energy in the batteries' electrolytes. These molecule receive and send out electrons, or negatively charged particles. Their electrochemical properties can be significantly affected by the choice of supporting electrolyte.
“The battery fluids also contain supporting electrolytes, which are salt solutions that conduct electricity between the battery’s positive and negative terminals,” the academics said. “The salts move across a membrane between the tanks to balance the charge or discharge of the redoxmers.”
In order to enable the monitoring of RFB performance in real time, carbon-based redoxmer molecules can be used as energy carriers and also to signal a problem known as “crossover.” This issue occurs when the redoxmers migrate to the wrong side of the battery.
“In this case, it’s particularly challenging, because we’re dealing with very small molecules dissolved in an electrolyte and with membranes that are porous,” said researcher Lu Zhang, noting that battery performance is considerably affected when the redoxmers penetrate the membrane between the two tanks.
The Argonne scientists have proposed one promising anolyte molecule for non-aqueous organic redox flow batteries, known as 2,1,3-benzothiadiazole (BzNSN), because it can be dissolved in a solvent-based electrolyte. At the same time, it can be engineered to fluoresce under ultraviolet light.
“These properties make it an excellent candidate for a self-reporting agent on the health within some types of flow batteries,” they explained.
The scientists conducted fluorimetry measurements at the DOE's Center for Nanoscale Materials to analyze the fluorescence of the BzNSN molecules in common flow battery fluids. Their tests have shown that these molecules exhibit different behaviors, depending on the electrolyte salts used. The electrochemical stability measurements have also shown that one BzNSN molecule configuration, in particular, was able to retain electrochemical function and stability over periods of days when in its charged state.
The scientists also conducted another experiment which showed that the fluorescent glow of the molecule, under ultraviolet light, can indicate how the molecule movements change, depending on the electrolyte composition.
“The fluorescence detection offers a great advantage over other methods because it’s highly sensitive,” said researcher Lily Robertson. “We see the molecule the minute it crosses over.”
They described their findings in “Fluorescence-Enabled Self-Reporting for Redox Flow Batteries,” which was recently published in ACS Publications.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.