Amid intense global competition to identify optimal battery chemistries, lithium-oxygen has stirred interest due to its reported potential to offer ten times more energy density than lithium-ion devices.
The claims of the energy storage tech are still undermined, however, by poor cycling stability caused by unstable molecules recombining into heterogeneous and unstable particles, prompting devices to degrade.
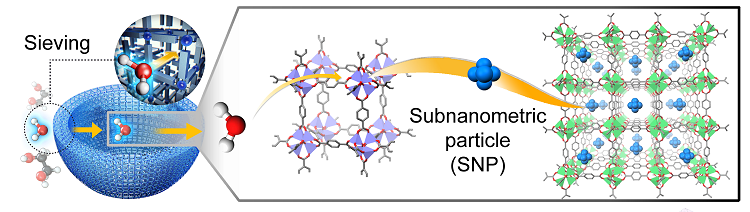
A research group including Jeung Ku Kang, of the department of materials science and engineering at the Korea Advanced Institute of Science and Technology (KAIST), set out to address the problem. In the paper Autogenous Production and Stabilization of Highly Loaded Sub-Nanometric Particles within Multishell Hollow Metal-Organic Frameworks and Their Utilization for High Performance in Li-O2 Batteries, published in Advanced Science, the researchers have explained how they devised sub-nanometric particles (SNPs) of atomic cluster sizes within metal-organic frameworks to improve stability under high-mass loading.
Breakthrough
The stabilization of SNPs through agglomeration on the back of their unsaturated surface bonds was only previously viable at lower mass loadings, where their collision frequency could be controlled. The pyrolysis of organometallic compounds induces the formation of heterogeneous SNPs at non-uniform particle sizes.
By alternating water-decomposable and water-stable metal-organic frameworks (MOFs), the researchers demonstrated, shells are built into a multi-layered MOF and isolated water molecules are then sieved through the hydrophobic nanocages of water-stable MOFs. Controlled hydrogen bonding affinity enables the creation of multi-shell hollow MOFs which allow for higher stability and conductivity. The researchers say the properties of MOFs with up to five shells lead to high electrochemical performance, including high volumetric capacity and lower over-potentials in Li-O2 batteries.
“Simultaneously generating and stabilizing atomic-level electrocatalysts within MOFs can diversify materials according to numerous combinations of metal and organic linkers,” said Kang. “It can expand not only the development of electrocatalysts but also various research fields such as photocatalysts, medicine, the environment and petrochemicals.”
The KAIST group found, for battery application, after MOFs had been treated with isolated water molecules they reacted with cobalt ions to form di-nuclear cobalt hydroxide. It was also possible to stabilize the cobalt hydroxide at the atomic level inside the sub-nanometric pores. Doing so enabled a 63.9% reduction of over-potential in the battery, which drove a tenfold increase in the device’s life-cycle.
Thanks to their high energy density, lithium-oxygen devices could bring real improvements to electrical vehicle batteries but further research is needed to bring them to market maturity.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.