Researchers from universities across Europe have discovered a way to greatly increase the efficiency of water electrolysis, potentially doubling the amount of hydrogen produced during the process.
‘Green hydrogen’ – electrolysis powered by renewable energy with zero carbon emissions – is touted by many as a vital part of the energy transition, either as a transport fuel or long term electricity storage option. However, renewable energy electrolysis is still pricey compared to the same process when powered by fossil fuel and the fate of green hydrogen will hang on continuing clean energy price falls, according to some analysts.
Reducing the amount of energy required to perform hydrolysis, of course, would be another way of cracking the nut.
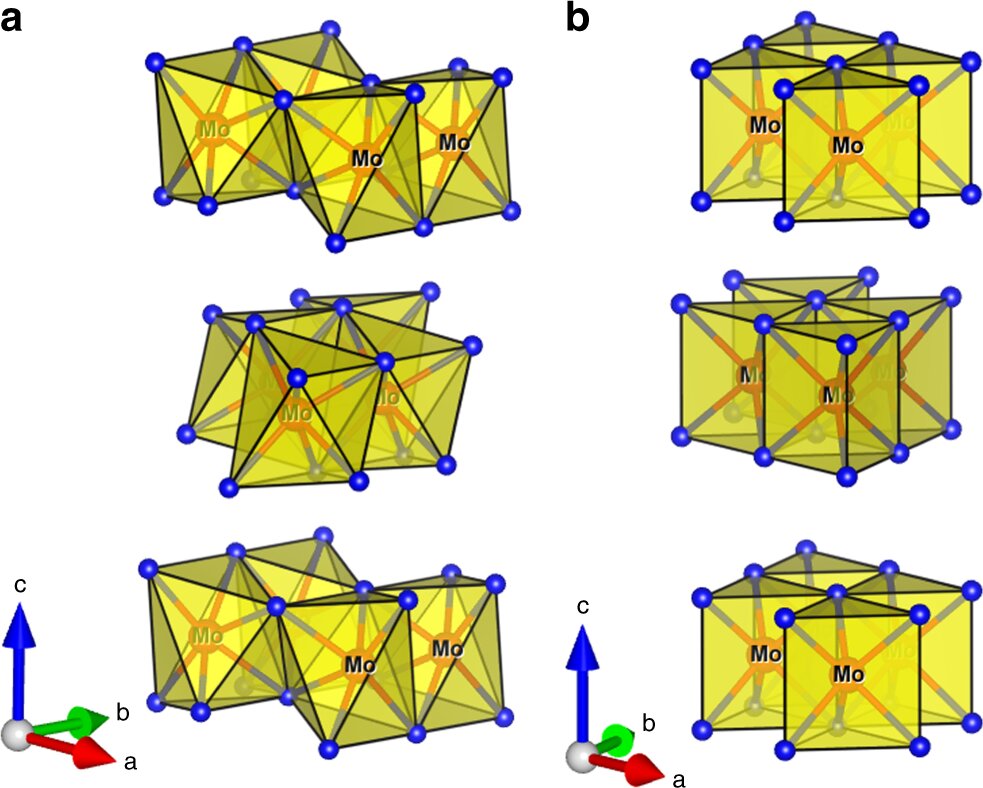
The European research team, led by Scotland’s University of Glasgow, worked with electrodes coated in a molybdenum telluride catalyst and found that when electricity was applied to the electrodes in a particular pattern of high-current pulses, the amount of hydrogen produced during electrolysis increased. By optimizing the pulse pattern, the team said it was able to almost halve the amount of electricity needed to produce a given amount of hydrogen.
Machine learning
The results, published in Nature Communications, show the performance of the catalyst was greatly improved when the electrode was held at a cathodic bias – reducing the overpotential needed to maintain a 10 milliamp cm−2 current from 320 millivolts to 178.
“It’s vital that we develop a robust suite of methods to store the energy for later use,” said Alexey Ganin, of the University of Glasgow, who led the team. “Batteries are one way to do that but hydrogen is a very promising alternative. Our research provides an important new insight into producing hydrogen from electrolysis more effectively and more economically, and we’re keen to pursue this promising avenue of investigation.”
The researchers posited machine learning could be used to further optimize the application of current pulses to achieve maximum output. They said the next stage of their research will focus on developing “an artificial intelligence protocol to replace human input in the search for the most effective electronic structures use in similar catalytic processes”.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



This article suggests that with the new technology, double the amount of hydrogen can be produced with the same amount of energy. This is not true. The same size of electrolyzer can produce double the amount of hydrogen, but only when it also uses double the amount of energy.
Dear Bas,
Thanks for your comment. I think you are mistaken here though.
“By optimising the pulses of current through the acidic electrolyte, they could reduce the amount of energy needed to make a given amount of hydrogen by nearly 50%.”
The above is a direct quote from the University of Glasgow’s announcement. I’m not a chemist, so my understanding of the paper they published is limited. But the group is certainly claiming to have reduced the energy requirement for producing hydrogen.
Excellent article!
As producer of renewable energy, we are very interested by any new development linked with H2 for long term storage.
• Per mole water splitting requires at least 241.8 kJ of energy. (Fixed by the laws of physics)
• Whichever method (thermal, electrolysis etc.) is followed, the energy required to split water will be always the same. When using electrolysis, it may be convenient to express it in eV (electron volts). The minimum band gap* for successful water splitting at pH=0 is 1.23 eV, practical of 2.3-2.4eV.
* band gap refers to the energy difference between the top of the valence band and the bottom of the conduction band. Electrons are able to jump from one band to another. However, an electron needs a certain amount of energy to jump from a valence band to a conduction band.
• https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201600337
https://en.wikipedia.org/wiki/Photocatalytic_water_splitting
https://pubs.acs.org/doi/pdfplus/10.1021/acsenergylett.6b00391
• “This is 241.8 — 260 kJ per mole of water or just shy of 5 eV per molecule of water (4 electrons times 1.23 V). Splitting one litre of water into hydrogen and oxygen would therefore take at least 16 MJ (4.4 kWh)”.
From: https://www.thenakedscientists.com/forum/index.php?topic=70430.0
From
From
• Best real-world estimates are that about 50kWh is required to produce a kilogram of hydrogen by electrolysis. (Note that this would amount to an opportunity cost of approximately $13 of electricity to produce 1kg of hydrogen at retail electricity rates—electricity that could have been used elsewhere (opportunity cost))
https://en.wikipedia.org/wiki/Liquid_hydrogen
• To produce 1 kg of hydrogen requires 8 litres of water and 41.4 kWh/kg of hydrogen. That’s 149 MJ/kg hydrogen. Hydrogen contains about 143 MJ/kg of energy. So, in the best possible scenario, under this model, the net outcome of return on energy invested using electrolysis to produce hydrogen is approximately -6MJ per kg—that is you consume 6MJ/kg more energy than the energy that is contained in the hydrogen that is produced.
Help me understand how this new technology proposes to overcome this?
Hi Rob,
Did you read the paper? There is a link to it in my article. The group worked with molybdenum telluride as a catalyst, which they found reduces the additional energy (the overpotential) needed to power the splitting reaction.