Scientists at California’s Stanford University have demonstrated a new setup for water electrolysis, which can produce hydrogen using saltwater.
While splitting water using electricity to produce hydrogen is a well-established technology, commercial applications for which are beginning to emerge in various markets, most electrolyzers require purified water to operate, and system components degrade quickly in the presence of salt.
Were the technology to be scaled up to represent a significant part of our energy mix, this could put a heavy strain on water resources. According to Stanford researchers, the amount of hydrogen required in scenarios where it is used to fuel vehicles and power cities could not be produced using purified water, but this problem could be solved if all the water in the sea were available. “Seawater is the most abundant aqueous electrolyte feedstock on Earth,” reads the research paper’s abstract. “But its implementation in the water-splitting process presents many challenges, especially for the anodic reaction.”
Key to the system demonstrated by Stanford is a new type of anode, which is protected against salt corrosion by a negatively charged layer. The researchers explain that the anode consists of a nickel foam core, which transports electricity from the power source, and nickel-iron hydroxide layered on top of nickel sulfide–nickel iron hydroxide sparks the electrolysis process, while the nickel sulfide becomes negatively charged during the process, protecting the core materials from chlorides in the saltwater. The researchers explain the concept as similar to how the negative ends of two magnets repel each other.
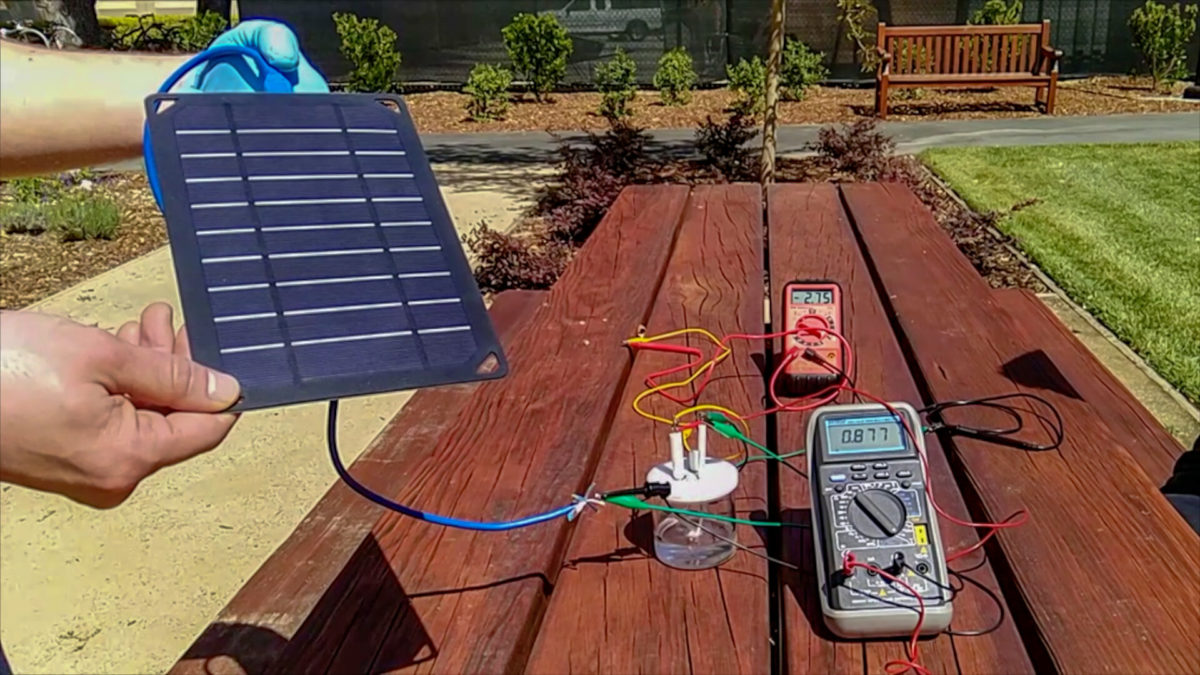
One experiment conducted by the group, described in the paper Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels, published in the journal Proceedings of the National Academy of Sciences of the United States of America, used a solar-powered demonstration machine to produce hydrogen and oxygen from seawater collected from San Francisco Bay. The electrolyzer operated at a current density of 400 mA/cm2 under a voltage of 2.12 continuously for more than 1,000 h without obvious decay.
The researchers note that without the coating, the anode usually falls apart after less than 12 hours of operation … the whole electrode falls apart into a crumble, but with this layer, it is able to go more than a thousand hours,” explained co-lead author on the paper Michael Kenney. “The impressive thing about this study was that we were able to operate at electrical currents that are the same as what is used in industry today.”
Scaling up
Stanford Chemistry Professor Hongjie Dai pointed out that his lab had shown proof of concept for the technology with this demonstration, but that scaling and mass production would be left to industrial players.
He went on to point out, however, that anodes incorporating the protective layer could be incorporated into existing electrolyzers, greatly easing the adoption of the technology. “It’s not like starting from zero,” Dai explains. “It’s more like starting from 80 or 90%.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.