A new solar water-splitting cell developed jointly by research institutes has demonstrated a record efficiency of 19.3%. The combination of a tandem solar cell of III-V semiconductors with a catalyst of rhodium nanoparticles and crystalline titanium dioxide drove the increase in efficiency. Researchers claim the cell can be used to form hydrogen from water directly, by immersing the cell in an aqueous medium.
Scholars from Helmholtz Zentrum Berlin, the University of Cambridge, the California Institute of Technology (Caltech), TU Ilmenau and Fraunhofer ISE have presented their research on increasing the efficiency of cells used to convert water into hydrogen. The team explained, the combination of solar cells with catalysts and a monolithic photoelectrode simplifies water splitting.
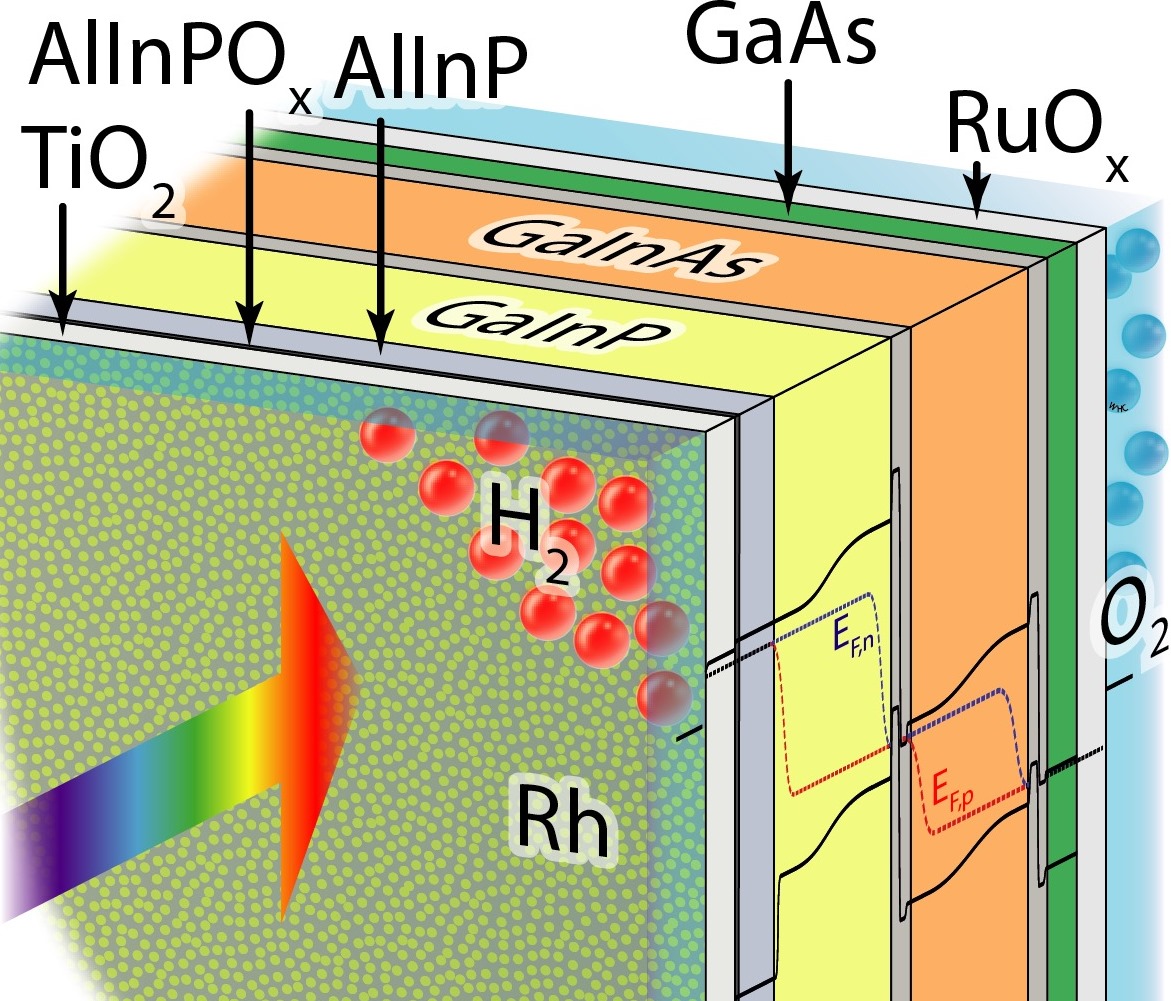
“The crystalline titanium-dioxide layer not only protects the actual solar cell from corrosion, but also improves charge transport, thanks to its advantageous electronic properties,” says Dr. Matthias May. “We were able to increase the operating life to almost 100 hours. This is a major advance compared to previous systems, that had already corroded after 40 hours. Nevertheless, there is still a lot to be done.”
Fraunhofer ISE provided the high-efficiency tandem cell which allowed the team to reduce the cell's surface reflectivity. “This is also where the innovation lies,” explains Caltech's Prof. Hans-Joachim Lewerenz. “Because we had already achieved an efficiency of [more than] 14% for an earlier cell in 2015, which was a world record at the time. Here we have replaced the anti-corrosion top layer with a crystalline titanium dioxide layer that not only has excellent anti-reflection properties, but to which the catalyst particles also adhere.
Fellow Caltech researcher Prof. Harry Atwater adds: “In addition, we have also used a new electrochemical process to produce the rhodium nanoparticles that serve to catalyze the water-splitting reaction. These particles are only ten nanometres in diameter, and are therefore optically nearly transparent, making them ideally suited for the job.”
The project partners highlight the importance of hydrogen production using renewable energy. To date, hydrogen production from renewables posts comparatively low efficiency. Higher efficiency cells capable of directly splitting water could be a way of overcoming this hurdle.
“This work shows that tailor-made tandem cells for direct solar water-splitting have the potential to achieve efficiencies beyond 20%,” says Prof. Thomas Hannappel, of TU Ilmenau. “One approach for this is to choose even better band-gap energies for the two absorber materials in the tandem cell. And one of the two could even be silicon.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



The competition for renewable hydrogen is electricity from PV or wind, then conventional electrolysis. This may be inefficient, but the first leg now enjoys enormous economies of scale. With LCOEs at 3c/kwh and future curtailed electricity at 0c/kwh, the economics will favour the indirect methods for some time.