Although lithium battery costs are falling, thanks to economies of scale are achieved through the electric vehicle industry, the cost of the technology remains high, and the batteries have some limitations in terms of their use to boost renewable energy consumption.
Now, a team of scientists at MIT has developed a battery which uses cheaper, abundant materials, and could be used for both short and long-term energy storage. The researchers estimate that the total chemical cost of the battery could be as little as 1/30 the cost of current storage technologies, including lithium ion.
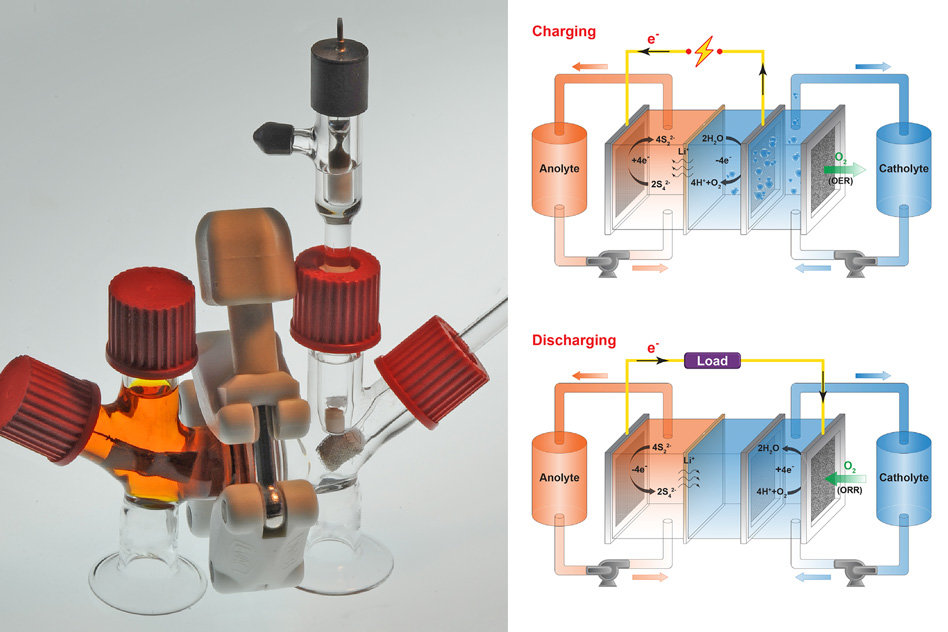
The battery utilizes a sulfur anode dissolved in water, and an aerated liquid salt solution in the cathode. Oxygen flowing in and out of the cathode causes the battery to discharge and charge.
The researchers chose to work with sulfur as it is an energy dense material which is both cheap and widely available. The challenge then was to find a suitable liquid cathode material in a similar price bracket.
A compound called potassium permanganate was known to be suitable for discharging energy, but the reduction reaction is not usually reversible, meaning the battery would not be rechargeable. In this case, however, an unexpected oxygen reaction in the cathode allowed the battery to charge, and the researchers were able to develop this into a working flow battery.
“This battery literally inhales and exhales air, but it doesn’t exhale carbon dioxide, it exhales oxygen,” explains Yet-Ming Chiang, co-author of the paper describing the battery, published in the journal Joule. “What this does is create a charge balance by taking oxygen in and out of the system.”
The current prototype is ‘about the size of a coffee cup’, however, flow batteries are known to be easily scalable, and Chiang states that cells could be combined into larger systems. He goes as far as to say that, thanks to its low materials cost, the battery could be the first technology to compete in cost and energy density with pumped hydroelectric storage.
Chiang also states that the battery has a slow discharge rate, and could therefore be used in seasonal storage – an increasingly important concept as solar moves into regions further from the equator, where sunlight levels vary more greatly between seasons.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



Dear Dr Chiang team…
I would LOVE to know more about the Sulfur – KMnO4 liquid battery.
I’m a retired Elec Mechanical engineer in South Africa.
I need to build an electricity storage device of some sort in the next year, and LI Ion is just too expensive.
I’d love to build a model of your invention, but I’m struggling to find out enough detail. Especially the precise chemistry and concentrations of the two sides and what is special about the internal electrode / membrane….??
The Oxygen exchange membrane I’d be inclined to make horizontal and in many very thin layers so that there can be direct air – liquid contact.??
Is it secret ? If not, could you please advise me where I can get information.
Regards
Martin